Is Receiving Tainted Blood From COVID-19 Vaccination or Infection an Irrational Fear?
EDITOR’S SUMMARY: Examining blood donation and recipient options for the unvaccinated medical freedom community is new territory; now highlighted post-COVID-19 pandemic mayhem. This concerned population also includes vaccinated folks who opted out of recent mRNA injections due to lack of proper testing, mistrust in the medical and/or political systems, medical reasons, and personal or religious beliefs. Investigating this issue is indeed a worthwhile endeavor.
By Janey Bibolet Ward
Increasing requests for COVID-19-free blood: There is a growing movement to establish blood banks with “directed donations” of unvaccinated blood. Recipients could match with donors who provide the blood directly to them for their treatment or upcoming procedure. In August, 2021, Kaiser Health News reported:
“We are definitely aware of patients who have refused blood products from vaccinated donors,” said Dr. Julie Katz Karp, who directs the blood bank and transfusion medicine program at Thomas Jefferson University Hospitals in Philadelphia.”
Vice News November 2022 article, “‘SafeBlood’ Hardliners Want to Set Up Unvaccinated Blood Banks,” reports that a growing number of people worldwide are demanding an investigation into the safety of donor blood after the worldwide COVID-19 exposure. The following groups have emerged and are registering donor members:
- Safe Blood Donations
- Blessed By His Blood
- OneBlood
- ABBA: Autologous Blood Banking of America
- Pure Blood Ministry
- IronHeart Bloodworks
Side effects from the COVID-19 vaccination related to blood and the heart: Definitive evidence proves that the COVID-19 vaccination impacts the blood and heart, and can lead to sudden adult death (SAD), stroke, myocarditis, brain inflammation, and progressive neurological disorders. Blood events that may occur include:
Blood Shortages During The Shutdown
In January 2021, the Red Cross, which supplies 40% of the nation’s blood supply, issued a press release: “The Red Cross Declares the First-ever Blood Crisis Amid the COVID-19 Omicron Surge,” sounding the alarm on blood donation shortages in the national blood banks.
As a result of the years-long COVID-19 pandemic and mass shutdowns, the nation is facing a critical blood shortage due to expired donations and the difficulty of restarting donor drives.
Because of the restrictions during the lockdown, blood banks were closed for many months, and critical time elapsed when no new blood came in. This is a problem, as blood banks must continually be replenished with repeated donations. It is critical to employ a screening program operating at full capacity to ensure the availability of safe donor blood in cases of emergency.
The Centers for Disease Control and Prevention (CDC) reported in December 2022, that approximately 68.9% of the United States population is estimated to have received the primary COVID-19 vaccine series (2 doses of Pfizer, Moderna, Novavax, or 1 dose of Johnson & Johnson).
Food and Drug Administration (FDA) And Centers for Disease Control and Prevention (CDC) Claim the Blood Supply Is Safe
In January 2021, the FDA and CDC announced the blood from donor banks was considered safe, and stated definitively:
“Respiratory viruses, in general, are not known to be transmitted by blood transfusion. There have been no reported cases of transfusion-transmitted coronavirus, including SARS-CoV-2, worldwide.”
In addition, “the FDA does not recommend using COVID-19 laboratory tests to screen routine blood donors.”
A May 2021 article in Transfusion, titled Minipool testing for SARS-CoV-2 RNA in United States blood donors, reported “when tested, no infectivity was observed in inoculated permissive cell cultures.”
January 2022 from the FDA: “Updated Information for Blood Establishments Regarding the COVID-19 Pandemic and Blood Donation,” stated that screening is not recommended for COVID-19-vaccinated blood.
It is said to be safe with no evidence to suggest an increased risk of adverse events nor any reported incidences of harm. Donors are to be observed for symptoms. If COVID-19 is suspected, the donor should be tested and prevented from donating for at least 10 days after testing positive and even if asymptomatic.
There is concern for transmissibility if these considerations are the guidance. Yet, recipients of “non-replicating, inactivated,” COVID-19 mRNA vaccines are cleared to donate without additional screening or testing.
Currently, the FDA requires the screening of donor blood to include multiple viruses and infectious agents, but not for COVID-19. There is no official protocol for identifying COVID-19 antibodies or infection, and screening is currently not a requirement.
Donors are asked about physical symptoms and infection within 14 days of the scheduled donation. A donor can test in commercial labs prior to giving blood, but is not required to do so.
Recipients of donor blood have no way of knowing if the lifeblood they are receiving can be definitively tested for any evidence of the mRNA gene therapy.
The control group, or unvaccinated for this series, is an important and statistically relevant demographic. There is a significant demand for the nations’ blood supply to be tested and monitored for COVID-19.
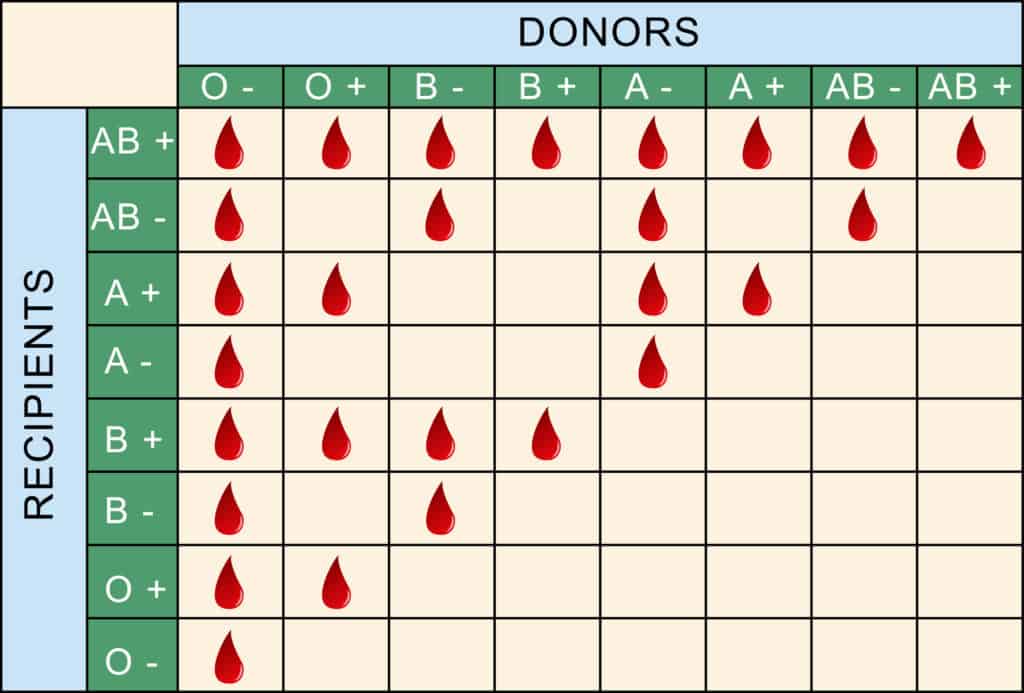
Blood Types and Donation Recipients
Determining donor-recipient matching is complex, and mixing blood types can be fatal due to clotting and incompatibility.
According to WebMD:
“What makes your blood different from someone else’s is your unique combination of protein molecules, called antigens and antibodies. Antigens live on the surface of your red blood cells. Antibodies are in your plasma. The combination of antigens and antibodies in your blood is the basis of your blood type.”
You are born with an inherited blood type from your parents. You have approximately 8-10 pints of blood circulating within your body. In an emergency, you may need a blood transfusion to sustain life. When planned surgeries are scheduled, you may require an infusion when blood loss is anticipated. Other types of medical issues such as blood disorders or cancer treatments will likely require donor blood.
The Red Cross is one of the leading blood banks in the United States. One pint is collected during donation and processed through a series of steps to prepare for use in transfusions:
- “Most whole blood donations are spun in centrifuges to separate it into transfusable components: red cells, platelets, and plasma.
- Plasma may be processed into components such as cryoprecipitate, which helps control the risk of bleeding by helping blood to clot.
- Red cells and platelets are leuko-reduced, which means your white cells are removed in order to reduce the possibility of the recipient having a reaction to the transfusion.
- Each component is packaged as a “unit,” a standardized amount that doctors will use when transfusing a patient.”
The Red Cross and other AABB (Association for the Advancement of Blood & Biotherapies) regulated blood donation centers require deferrals for some medications, immunizations, vaccinations and medical conditions. The FDA is tasked with inspecting facilities every two years.
Blood banks routinely test for infectious agents. Additional testing includes screening for transmission of prion-associated diseases, blood group and blood group antibodies, and molecular blood group testing.
A 2014 report from the Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee (JPAC) states:
“Routine DNA testing/genotyping using rapid automated technology is likely to enter blood service and hospital laboratory practice in the next decade.”
A 2018 study, Establishment of Reference Panels for Blood Group Genotyping Tests, is working to develop reference panels designed to include approximately 90 genotypes of the blood group systems that will be made available to blood typing device manufacturers and testing laboratories.
Two Ways to Ensure a Secure Blood Donation
Autologous blood banking: Store your own blood for planned surgery, an emergency, or a transfusion.
Directed or designated donor blood: Find a match, designate a selected donor and store the blood. Specific forms must be completed in advance of the donation and recipients should prepare weeks in advance of any scheduled surgery.
Designated donor (donor is personally requested by the recipient or recipient’s family) requests can be challenging, especially in an emergency, as they may take several weeks to coordinate and process. Blood can safely be stored in blood banks and ordered by hospitals for a very limited time, therefore timing is critical and precarious for pending recipients. The Red Cross cautions that receiving a blood transfusion from a blood relative may increase your risk of alloimmunization—an immune response to foreign antigens that may result in complications.
In the COVID-19 pandemic, recovered patients’ plasma was used to treat infected patients, known as “passive immunization,” and was thought to confer the recipients with a greater chance of recovery and survival from the novel virus.
An August 2021 National Institutes of Health (NIH) study, “Early treatment with convalescent plasma for COVID-19 doesn’t show benefit,” disputed this treatment worked.
A Case of Medical Kidnapping for Refusing COVID-19 Transfusion
In 2022, a family in New Zealand requested COVID-19-free donor blood for their newborn child, in advance of a life-saving heart surgery. Fearful of blood clots or rejection issues, the family petitioned to use specific donor blood for their infant son.
The case was taken to court, and the judge ruled against the parents. Custody of the child was transferred to the state until the procedure was conducted.
“In his judgment, Justice Ian Gault stated that the parents’ proposal is not supported by peer-reviewed articles, yet the affidavit submitted by Dr. Byram Bridle references a peer-reviewed article by Bansal et al. showing that spike protein is found on exosomes in the blood for at least four months after the second Pfizer injection. Covid-19 injections remain experimental and giving blood containing these experimental vaccine products to an infant is a clear violation of the Nuremberg Code.”

The Case of Baby Alexander Bly
Baby Alexander was born in January 2022 and required emergency surgery to correct a fistula in his throat as well as a heart defect. His parents requested donor blood from a person not vaccinated with the emergency authorized, experimental mRNA COVID-19 biologic, for fear of rejection and potential blood clotting.
Following his first surgery he was given a transfusion without his parents permission, developed blood clots that did not respond to blood thinners, and died on February 17, 2022. They are currently pursuing legal action against the hospital.
The Urgent Call to Monitor Transfusions in the COVID-19 Era
At this time it is unknown if donors vaccinated with COVID-19 are safe donors. It is essential that donor blood be required to be tested and screened for COVID-19 vaccine antibodies, in addition to the infectious agents in the current protocol.
It’s not just blood transfusions to watch out for, but rather, all blood-derived products. For example, RhoGAM, given to pregnant women to prevent complications, and IgG, administered via IV or subcutaneously to fight infections.
The rise of new internationally-forming blood banks provide potential options for securing blood, free from potential contamination from the mRNA injections. Investigate the options provided for your family in advance of needing donor blood for an unforeseen emergency or planned procedure or surgery.
Disclaimer: A Voice For Choice Advocacy does not endorse the use of any particular blood bank. It is up to you, the consumer, to research the effectiveness and safety protocols used by these organizations.